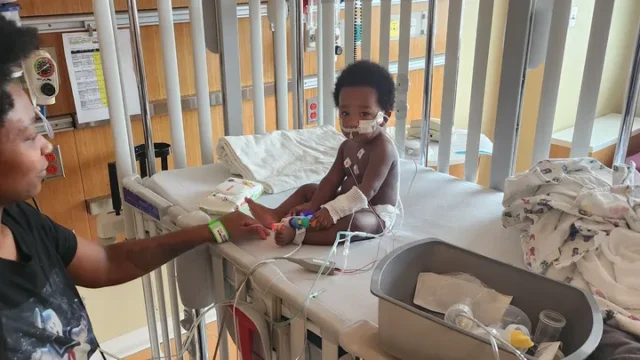
The CDC and FDA reported last Friday that two samples collected by FDA tested positive for Clostridium botulinum type A.
One is a ByHeart powdered infant formula closed product sample that matches a clinical isolate from an infant included in this outbreak according to whole genome sequencing analysis (WGS).
The second whole milk powder sample was collected by FDA at a supplier to ByHeart and analyzed by the New York Wadsworth Laboratory. WGS analysis showed that the Clostridium botulinum found in the sample of whole milk powder is a genetic match to the Clostridium botulinum detected in the finished product sample of ByHeart’s infant formula, according to analysis conducted by ByHeart.
These samples also match two isolates of whole milk powder, an ingredient that ByHeart uses in the production of ByHeart Whole Nutrition powdered infant formula, which were collected and tested by ByHeart.
And, other samples have tested positive as well.
| Sample Collected/Analyzed by | Product | Test Result | Toxin Type |
| CDPH | Opened container of ByHeart Infant Formula (Batch No. 251131P2) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch 251261P2 or Batch 251131P2) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch 251261P2 or Batch 251131P2) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch 251261P2 or Batch 251131P2) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch 251261P2 or Batch 251131P2) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch 251261P2 or Batch 251131P2) | Positive | Type A |
| ByHeart | ByHeart Infant Formula (Batch 251261P2 or Batch 251131P2) | Positive | Type A |
| Collected by AZ/Analyzed under FDA LFFM | Unopened container of ByHeart Infant Formula (Batch No. 251481P2) | Positive | Type A |